Reactive molecular collisions
Imagine if you will a chemical reaction between carbon monoxide and nitrous oxide:
CO + N2O → CO2 + N2
In the total energy terms "E", e,t,r, and v stand for electronic, translational, rotational, and vibrational.
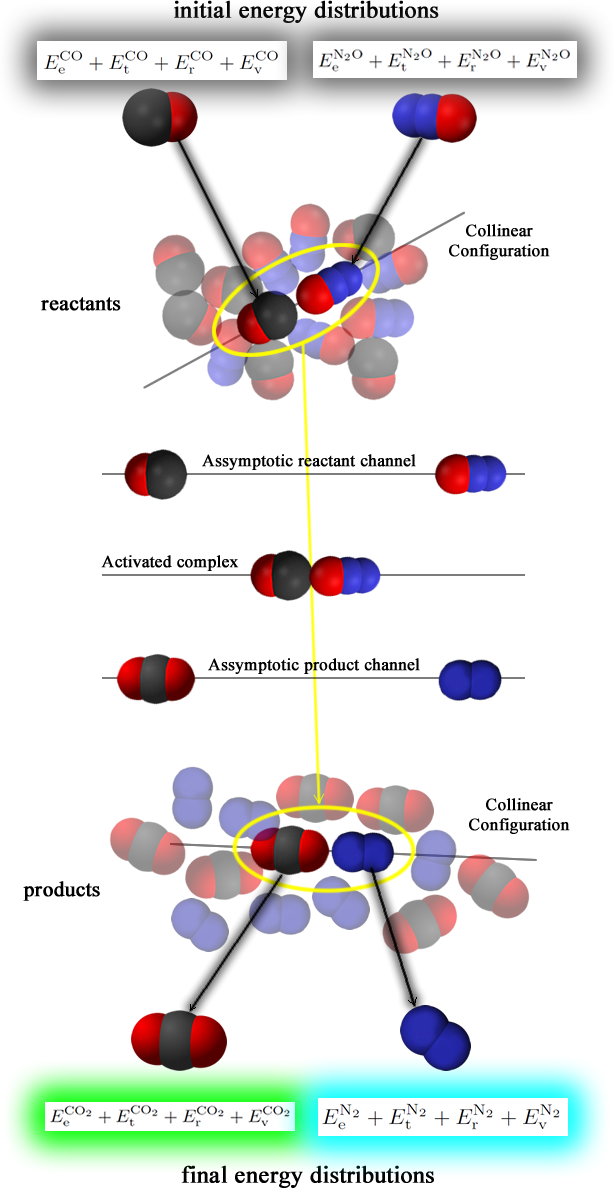
After the reaction, the density of each of these states changes. That is the essence of molecular reaction dynamics.
back to Including orbital angular momentum go to Including spectators
