Nonreactive molecular collisions
In nonreactive collisions, particles collide somewhere between elasticaly and inelastically. Elastic collisions would invovle no exchange of energy between the two colliding particles. Inelastic collisions do exchange energy, therefore some degrees of freedom lose or gain energy during an inelastic collision. In the total energy terms "E", e,t,r, and v stand for electronic, translational, rotational, and vibrational. Here is a diagram of the process: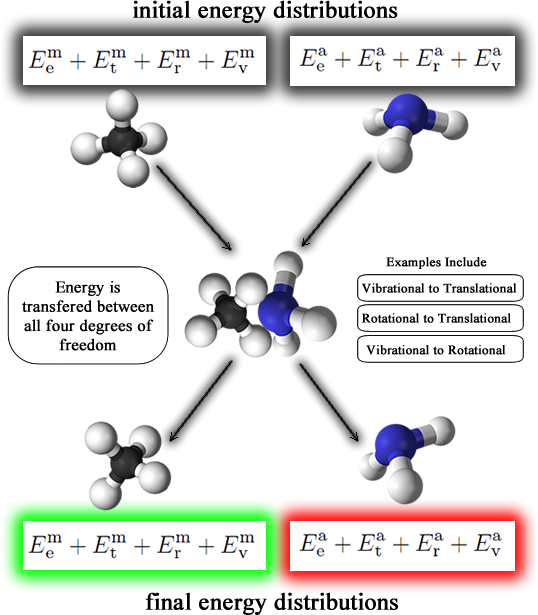
After the inelastic collsion, the density of each of these states changes. For example, the methane molecule could lose some translational energy to one of the normal vibrating ammonia modes.
back to Dipole field go to Including orbital angular momentum
